Atomic emission spectroscopy is the earliest generation and development of optical analysis methods.
Atomic emission spectroscopy is a method of determining the composition of a substance by using the atomic or ion emission characteristic spectrum of each element under thermal or electrical excitation, and performing qualitative and quantitative analysis of the elements.
(1) Advantages of atomic emission spectrum analysis:
It has the ability to detect multiple elements simultaneously. It can measure multiple elements in a sample at the same time.
The analysis speed is fast. Dozens of elements can be quantitatively analyzed simultaneously in a few minutes.
The detection limit is low. General light source can reach 10~0.1mg/mL, inductively coupled high frequency plasma atomic emission
The detection limit of ICP-AES can reach ng/mL level.
The accuracy is high. Generally, the relative error of light source is about 5%~10%, and the relative error of ICP-AES can reach less than 1%.
The sample consumption is low.
The linear range of the calibration curve of ICP light source is as wide as 4 to 6 orders of magnitude.
(2) Disadvantages of atomic emission spectrum analysis:
The accuracy of high content analysis is poor;
Common non-metal elements such as oxygen, sulfur, nitrogen, halogen and other spectral lines are in the far ultraviolet region. General spectrometers cannot yet detect;
Some non-metal elements, such as P, Se, Te, etc., have low sensitivity due to their high excitation potential.
It can only be used to determine the elemental composition and content of a substance, and cannot give information about the substance molecule.
Cannot make morphological analysis.
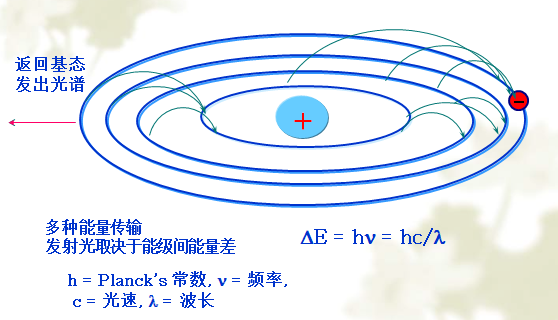
Generation of atomic emission spectra
Normally, the atom is in the ground state. Under the action of the excitation light, the atom gains enough energy, and the outer electrons transition from the ground state to a higher energy state, that is, the excited state. The atom in the excited state is unstable and its lifetime is less than 10-8s, and the outer electrons transition from a high energy level to a lower energy level or ground state. The excess energy is emitted in the form of electromagnetic radiation, resulting in an emission spectrum.
The spectrum produced in this way is a linear spectrum.
Service Hotline:13401198429(Manager Liu)
MORE